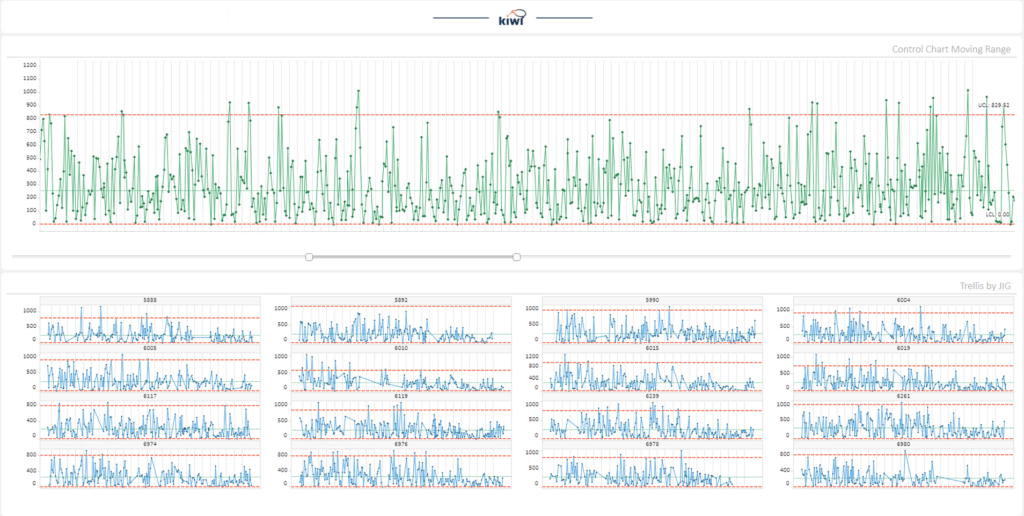
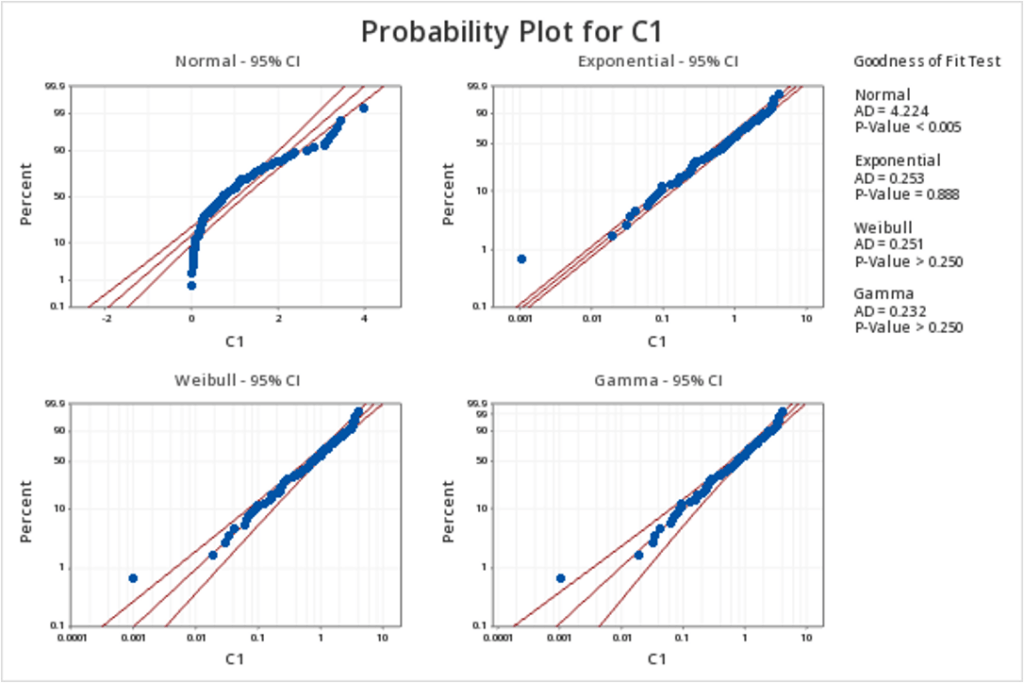
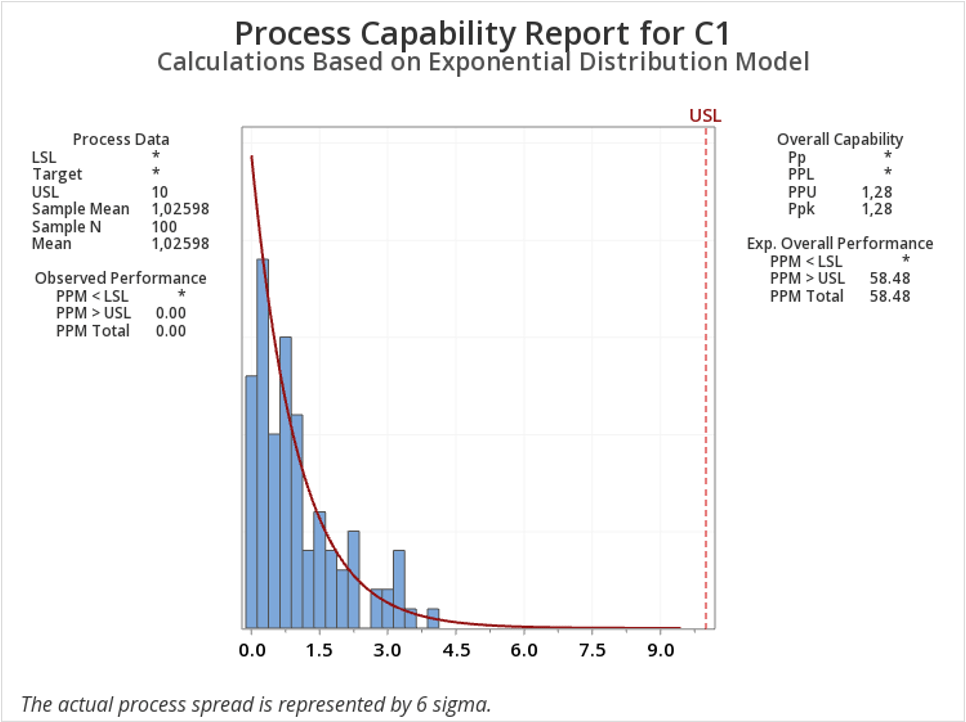
Empowering Pharmaceutical Excellence
Our comprehensive suite of services spans every stage of the pharmaceutical lifecycle, from Product Quality Review to formulation design, process optimization, and beyond.
In today’s dynamic pharmaceutical landscape, achieving excellence is not just a goal; it’s a necessity. Our pharmaceutical services division stands at the forefront, offering a diverse array of solutions meticulously crafted to meet the evolving needs of the industry. With a relentless commitment to innovation, compliance, and quality, we deliver unparalleled support to pharmaceutical companies worldwide. Our services are designed to empower you with the insights and tools necessary to drive innovation and achieve success in today’s competitive pharmaceutical landscape. Join us on a journey toward pharmaceutical excellence – where innovation, compliance, and quality converge to shape a brighter future for healthcare worldwide.